Project 1: Filopodia and Developmental Timing
How do Prothoracic Gland cells efficiently release Ecdysone hormone?
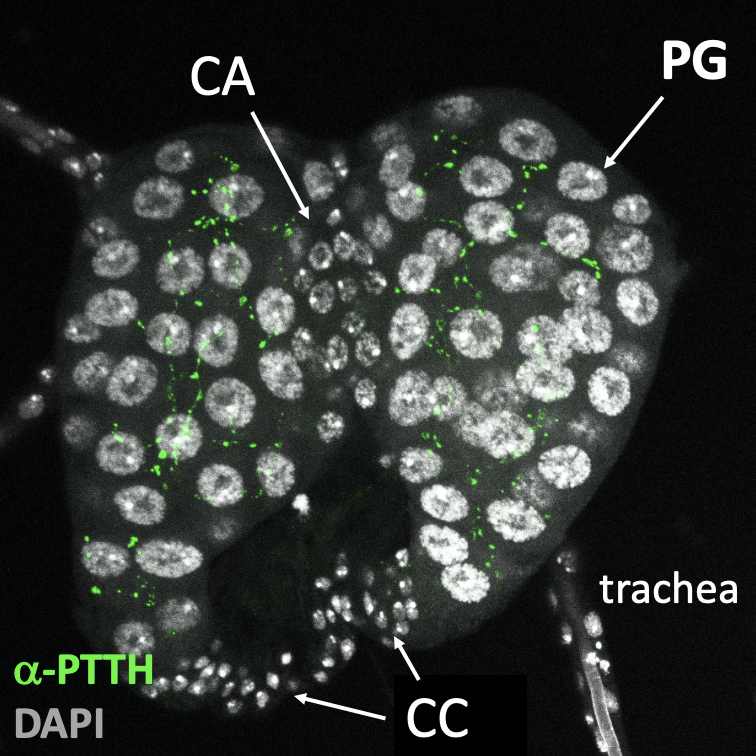
The Prothoracic Gland of Drosophila melanogaster
plays a pivotal role in the insect’s developmental progression, serving as the primary site for the
synthesis and release of ecdysone, a steroid hormone
essential for molting and metamorphosis. During larval stages, the Prothoracic Gland resides
within the Ring Gland (Figure 1) and is composed of
highly specialized polyploid cells that are finely tuned to environmental and developmental signals.
These cells utilize dietary cholesterol as a precursor for ecdysone
biosynthesis, processing it through enzymatic pathways governed by the Halloween genes. The ecdysone
produced is then secreted into the hemolymph, where it is transported to target tissues and converted
into its active form, 20-hydroxyecdysone (20E). This active hormone drives key developmental transitions
by coordinating tissue remodeling, cellular growth, and differentiation, ensuring proper progression
through life stages. The Prothoracic Gland not only integrates nutritional and hormonal signals but also
interacts with upstream regulators, establishing itself as a cornerstone for studying endocrine control
and systemic growth dynamics in insects. Read Delanoue and Romero, 2020 for review.
Central to the regulation of the Prothoracic Gland are the PTTH
(prothoracicotropic hormone) neurons (Figure
1), which serve as master regulators of developmental timing in
Drosophila. These neurons, located in the larval brain, secrete PTTH, a
neuropeptide that directly targets the PG to stimulate ecdysone production.
The activity of PTTH neurons is tightly controlled by a complex network of environmental and
physiological inputs. It has been shown that PTTH regulates environmental adaptive plasticity (Shimell
et al., 2018) and light avoidance in larvae (Yamanaka et al., 2013). By integrating these signals, PTTH
neurons ensure that ecdysone synthesis occurs at precisely the right moment to trigger molting and
metamorphosis. This regulatory system allows the organism to adapt its growth and maturation to
environmental conditions, linking external factors to internal endocrine pathways. Beyond their role in
timing, PTTH neurons exemplify how neural circuits orchestrate endocrine responses, making them a
critical focus for understanding the interface between environmental cues, neurobiology, and
developmental endocrinology.
For decades, it was widely believed that ecdysone, due to its lipophilic nature, diffused freely across
cell membranes. However, recent research has revealed that prothoracic gland (PG) cells release ecdysone
through a vesicle-mediated mechanism. Specifically, after being metabolized in
mitochondria, ecdysone is actively loaded into vesicles via the ATP-binding cassette (ABC) transporter
Atet. These vesicles are then transported to the plasma membrane, where their fusion is mediated by the
synaptotagmin protein Syt1, resulting in the regulated release of ecdysone into the hemolymph (Yamanaka
et al., 2015). Building on this groundbreaking discovery, our project aims to
elucidate the precise molecular and cellular mechanisms that enable prothoracic gland cells to
efficiently secrete this critical hormone.
This work was just published in Nature Communications!
